第10回夏のシンポジウム
「夏の国際シンポジウム」のご案内 (協賛:国際アミノ酸化科学協会 https://icaas-npo.org )
2024年の夏のシンポジウムは、特別企画「夏の国際シンポジウム」として台湾で開催予定です。
アミノ酸学会、台湾からそれぞれ3名、計6名の講師をお呼びしてアミノ酸・栄養に関連するトピックスをご講演いただきます。
会場:台湾台北市 台湾大学病院国際会議場
会期:2024年7月19日(金曜日)
7月19日夜には懇親会、7月20日には台湾大学病院訪問・エクスカーションを予定しています。
参加費:10000円
懇親会費:15000円
エクスカーション参加費:15000円
飛行機・ホテルは各自ご手配ください。
参加希望者は2024年5月1日2024年6月10日(月)までに、下記のアドレスに件名「アミノ酸学会夏のシンポ」とつけて参加登録情報をお送りください。
参加費・懇親会費・エクスカーション参加費は2024年6月18日までに下記口座に振り込みをお願いします(振込手数料は各自お支払いください)。
不参加となった場合の払い戻しはできませんのでご容赦ください。
メール宛先:実行委員長 深柄和彦 kazfukatsu@yahoo.co.jp
——–
参加登録情報
お名前(ふりがな):
ご施設:
役職:
メールアドレス:
連絡先住所:
連絡先電話番号:
シンポジウム参加の人数(同伴者も参加される場合は同伴者のお名前)
懇親会参加の有無: 参加の場合の人数と同伴者のお名前
エクスカーション参加の有無: 参加の場合の人数と同伴者のお名前
振込口座
みずほ銀行 本郷支店(店番号075) 口座番号 4222670 日本アミノ酸学会第10回夏のシンポジウム世話人 深柄和彦
——–
若手トラベルグラント申請について(35歳以下の研究者の方)
若手研究者の参加を支援するために、学生会員ならびに35歳以下の研究者にはトラベルグラント(5~10万円、渡航費の支援:支援額は応募総数により決定)を支給します。申請書の書式を本サイトに掲載します。6月10日までに学会事務局にお送りください jsaas@asas-mail.jp
グラント支給の可否は役員会で審議の上決定させていただきます。
シンポジウムプログラム
午前10時~午後5時
亀井康富 先生(京都府立大学)
白木伸明 先生(東京工業大学)
佐々木理人 先生(筑波大学小児外科)
Prof. Kuo Kung-Kai : Kaohsiung Medical University
Dr. Wu Jin-Ming:national Taiwan University Hospital
Prof. Hou Yu-Chen:Taipei Medical University
| 10:00-10:15 | Opening Remarks | Chair of the symposium Kazuhiko FUKATSU Surgical Center, The University of Tokyo Hospital |
|---|---|---|
| Co-chair Ming Tsan LIN Department of Surgery, National Taiwan University Hospital |
||
| President of Japanese Society of Amino Acid Sciences Shigeki FURUYA Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Kyusyu University |
||
| Symposium 1 | Moderator Yasuyuki KITAURA Department of Food and Nutritional SciencesCollege of Bioscience and BiotechnologyChubu University |
|
| 10:15-11:00 | Speaker Yasutomi KAMEI Laboratory of Molecular Nutrition, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University “Amino acids and skeletal muscle function” |
|
| 11:00-11:45 | Speaker Kung-Kai KUO Division of General and Digestive Surgery, Department of Surgery “Alternations in Amino Acids and Lipid Metabolic Pathways in Cancer Stem Cells” |
|
| 11:45-13:15 | Lunch | |
| Symposium 2 | Moderator Asako TAKENAKA School of Agriculture, Meiji University |
|
| 13:15-14:00 | Speaker Takato SASAKI Pediatric Surgery, University of Tsukuba Hospital “Plasma L-citrulline levels as indicators of residual intestinal function in pediatric patients with short bowel syndrome (SBS)” |
|
| 14:00-14:45 | Speaker Jin-Ming WU Department of Surgery, National Taiwan University Hospital “Dualism versus Monism in Surgical Nutrition” |
|
| 14:45-15:15 | Break | |
| Symposium 3 | Moderator Hisanori KATO Faculty of Nutrition, Kagawa Nutrition University |
|
| 15:15-16:00 | Speaker Hou Yu-CHEN School of Food Safety, College of Nutrition, Taipei Medical University “Effects of glutamine and leucine on muscle injury and atrophy in sepsis” |
|
| 16:00-16:45 | Speaker Nobuaki SHIRAKI School of Life Science and Technology, Tokyo Institute of Technology “Methionine and Zinc: Key Regulators of Pluripotent Stem Cell Differentiation” |
|
| 16:50-17:00 | Closing Remarks | Vice President of Japanese Society of Amino Acid Sciences Fumiaki YOSHIZAWA Department of Bioproductive Science, School of Agriculture, Utsunomiya University |
| 18:00 | Dinner | The restaurant is Guest House in Sheraton Hotel. 17F, NO 12, SEC 1, ZHONGXIAO EAST ROAD, ZHONGZHENG DISTRICT, TAIPEI CITY, TAIWAN Casual dressing is ok. No need to tie or formal dressing. |
|---|
| 8:45 | Entrance of Sheraton Hotel |
|---|---|
| 9:00 | Entrance of National Taiwan University Hospital |
エクスカーション(2024年7月20日・土)
【予定コース】
午前:ホテル~台湾大学病院視察~中正記念堂~昼食(鼎泰豊/台北101)
午後:故宮博物院見学~十份(老街散策、天燈上げ体験)~九份~台北市内にて夕食~士林夜市~ホテル
【ツアー代金に含まれるもの】
大型バス:1台(43名乗り)、日本語ガイド、食事(昼食:1回、夕食:1回)、
天燈上げ体験、故宮博物院入場料
【ツアー代金に含まれないもの】
お食事時の飲み物代、その他個人的な飲食・購入品
ホテルマップ
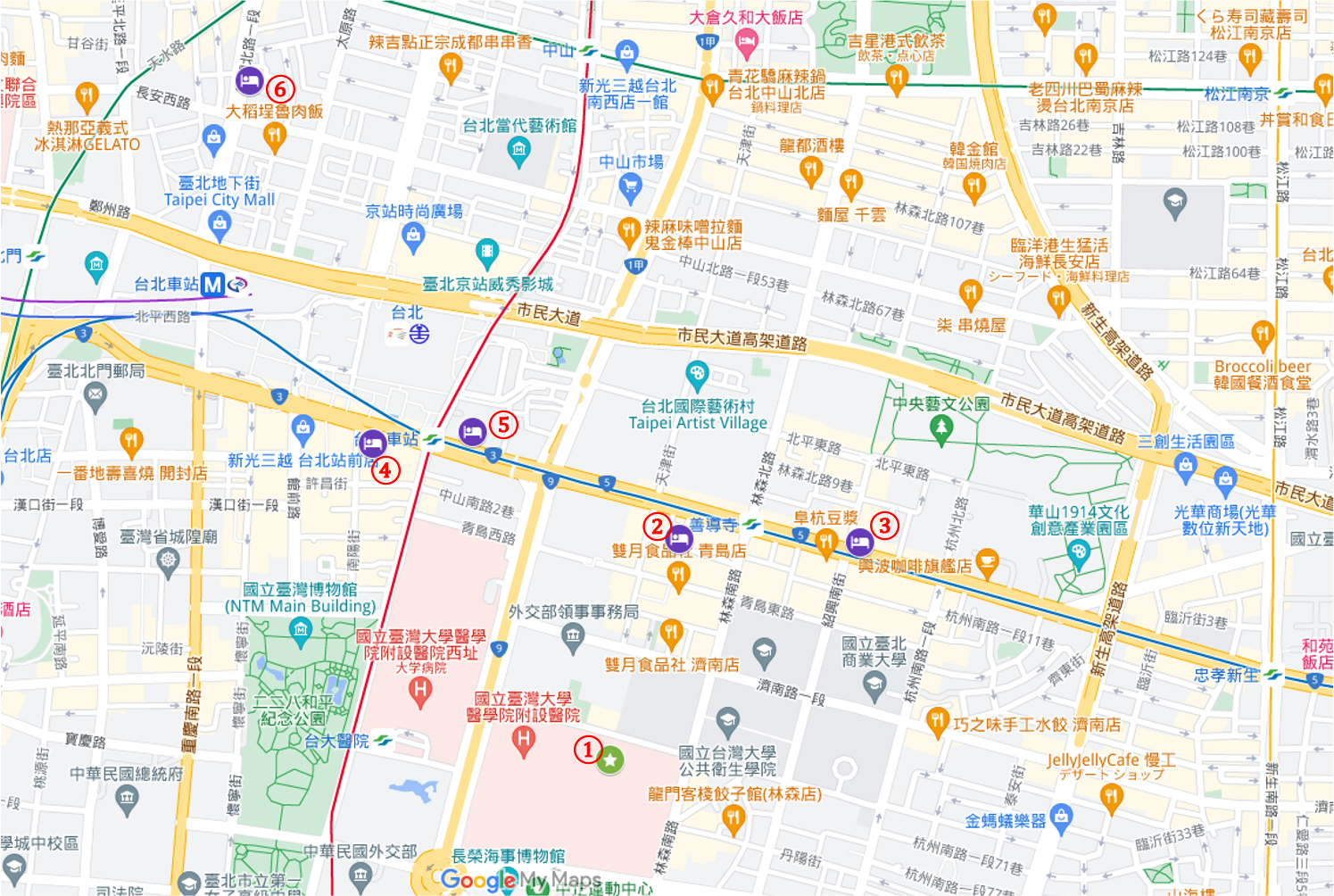
① 台湾大学医院国際会議センター
② シェラトングランド台北ホテル
③ ホテルコッツィ台北忠孝館
④ シーザーパークホテル
⑤ コスモスホテル台北
⑥ オーチャードパークホテル
JSAAS international summer symposium 2024
(Sponsorship: The International Council on Amino Acid Science, ICAAS https://icaas-npo.org/english)
Theme: Let’s discuss on amino acid sciences and nutrition therapy !
Venue: TAIWAN, NTUH International Convention Center
Date: 19th July, 2024 (10AM-5PM)
Chairperson: Prof. Kazuhiko FUKATSU, The University of Tokyo Hospital
Co-chairperson: Prof. Ming-Tsan LIN, National Taiwan University Hospital
Speaker:
Prof. Yasutomi KAMEI, Kyoto Prefectural University
Prof. Nobuaki SHIRAKI, Tokyo Institute of Technology
Dr. Takato SASAKI, University of Tsukuba Hospital
Prof. Kuo Kung-Kai : Kaohsiung Medical University
Dr. Wu Jin-Ming:National Taiwan University Hospital
Prof. Hou Yu-Chen:Taipei Medical University
Will be held under co-sponsorship of Taiwan Society for Surgical Metabolism and Nutrition
Will be supported by ICAAS
| 10:00-10:15 | Opening Remarks | Chair of the symposium Kazuhiko FUKATSU Surgical Center, The University of Tokyo Hospital |
|---|---|---|
| Co-chair Ming Tsan LIN Department of Surgery, National Taiwan University Hospital |
||
| President of Japanese Society of Amino Acid Sciences Shigeki FURUYA Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Kyusyu University |
||
| Symposium 1 | Moderator Yasuyuki KITAURA Department of Food and Nutritional SciencesCollege of Bioscience and BiotechnologyChubu University |
|
| 10:15-11:00 | Speaker Yasutomi KAMEI Laboratory of Molecular Nutrition, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University “Amino acids and skeletal muscle function” |
|
| 11:00-11:45 | Speaker Kung-Kai KUO Division of General and Digestive Surgery, Department of Surgery “Alternations in Amino Acids and Lipid Metabolic Pathways in Cancer Stem Cells” |
|
| 11:45-13:15 | Lunch | |
| Symposium 2 | Moderator Asako TAKENAKA School of Agriculture, Meiji University |
|
| 13:15-14:00 | Speaker Takato SASAKI Pediatric Surgery, University of Tsukuba Hospital “Plasma L-citrulline levels as indicators of residual intestinal function in pediatric patients with short bowel syndrome (SBS)” |
|
| 14:00-14:45 | Speaker Jin-Ming WU Department of Surgery, National Taiwan University Hospital “Dualism versus Monism in Surgical Nutrition” |
|
| 14:45-15:15 | Break | |
| Symposium 3 | Moderator Hisanori KATO Faculty of Nutrition, Kagawa Nutrition University |
|
| 15:15-16:00 | Speaker Hou Yu-CHEN School of Food Safety, College of Nutrition, Taipei Medical University “Effects of glutamine and leucine on muscle injury and atrophy in sepsis” |
|
| 16:00-16:45 | Speaker Nobuaki SHIRAKI School of Life Science and Technology, Tokyo Institute of Technology “Methionine and Zinc: Key Regulators of Pluripotent Stem Cell Differentiation” |
|
| 16:50-17:00 | Closing Remarks | Vice President of Japanese Society of Amino Acid Sciences Fumiaki YOSHIZAWA Department of Bioproductive Science, School of Agriculture, Utsunomiya University |
| 18:00 | Dinner | The restaurant is Guest House in Sheraton Hotel. 17F, NO 12, SEC 1, ZHONGXIAO EAST ROAD, ZHONGZHENG DISTRICT, TAIPEI CITY, TAIWAN Casual dressing is ok. No need to tie or formal dressing. |
|---|
| 8:45 | Entrance of Sheraton Hotel |
|---|---|
| 9:00 | Entrance of National Taiwan University Hospital |
Registration fee: 10,000 Japanese yen for attendees from Japan
20 US$ for attendees from Taiwan
Visit to National Taiwan University Hospital is scheduled on 20th July, 2024 for
Japanese attendees.
Application Period; to Monday, June, 10, 2024
Registration Information
Your name:
Affiliation:
Appointment:
Email Address:
Contact Address:
Contact Phone Number:
Number of people attending the symposium (if accompanying persons are also attending, please list names of accompanying persons)
Please send your registration information to the address (kazfukatsu@yahoo.co.jp) with the subject line “Amino Acid Society of Japan Summer Symposium”.
 Amino acids and skeletal muscle function
Amino acids and skeletal muscle function
Yasutomi Kamei
Laboratory of Molecular Nutrition, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University
(E-mail: kamei@kpu.ac.jp)
The skeletal muscle is the largest organ of the human body and plays important roles in exercise, energy expenditure and glucose/amino acid metabolism. Appropriate exercise coupled with sufficient nutrition results in an increase in muscle mass. In contrast, various conditions, such as bedrest, aging, cancer, and other diseases, lead to muscle atrophy, which decreases energy expenditure (leading to obesity), decreases glucose uptake and increases blood glucose (resulting in diabetes), and decreases quality-of-life.
We have been investigating the role of a transcription factor FOXO1 in metabolic regulation of the skeletal muscles. In particular, based on the observation that FOXO1 gene expression is induced by energy deprivation, we produced transgenic mice that overexpress FOXO1 and found that they exhibit muscle atrophy. In addition to FOXO1, transcriptional coactivators PGC1s are important for muscle metabolism. PGC1α expression increases in the skeletal muscles upon exercise, which leads to an increase in mitochondrial numbers in the skeletal muscles and red/slow fiber formation. Thus, we are investigating the detailed mechanisms of muscle metabolism, including amino acid metabolism, in vivo and in vitro, with focus on FOXO1 and PGC1α/PGC1β as key regulators. We hope that the mechanisms underlying the prevention of atrophy and dysfunction of the skeletal muscle will thereby be clarified, coupled to an increased understanding of the utility of functional foods for the prevention and/or alleviation of these conditions.
 Methionine and Zinc: Key Regulators of Pluripotent Stem Cell Differentiation
Methionine and Zinc: Key Regulators of Pluripotent Stem Cell Differentiation
Nobuaki Shiraki
Recent advances in biology heavily rely on cell culture technology. Basic and clinical research using cell culture is conducted in many fields, including drug discovery research, vaccine development, biopharmaceutical production, and regenerative medicine. In regenerative medicine, optimal differentiation media is required to differentiate target cells from stem cells efficiently. This medium consists of a basal media containing nutrients such as amino acids, sugars, vitamins, and trace elements, as well as growth factors and signal stimulants/inhibitors.
We have been developing differentiation methods focusing on basal culture media and reported that human Pluripotent stem cells (PSCs) require S-adenosylmethionine (SAM), a metabolite of methionine, to maintain their undifferentiated nature. Methionine deficiency in the medium reduced SAM in undifferentiated PSCs, altered their epigenetic state and thus rendering PSCs in a state potentiated for differentiation (Shiraki et al., Cell Metab 2014)(Ozawa et al., Stem Cells 2022).
This study finds that methionine deprivation triggers a reduction in intracellular protein-bound Zn content and upregulation of Zn exporter SLC30A1 in PSCs. Culturing PSCs in a Zn-deprived medium results in decreased intracellular protein-bound Zn content, reduced cell growth, and potentiated differentiation, which partially mimics methionine deprivation. PSCs cultured under Zn deprivation exhibit an altered methionine metabolism-related metabolite profile (Sim et al., Cell Reports 2022). We conclude that methionine deprivation potentiates differentiation partly by lowering cellular Zn content. We establish a protocol to generate functional pancreatic β cells by applying methionine and Zn deprivation (Sim et al., STAR Protoc 2023). The above statement strongly implies that there is a relationship between methionine metabolism and zinc signaling in the control of human iPS cell fate. In this presentation, I aim to introduce and discuss the connection between amino acid metabolism and trace elements in stem cells.
 Plasma L-citrulline levels as indicators of residual intestinal function in pediatric patients with short bowel syndrome (SBS).
Plasma L-citrulline levels as indicators of residual intestinal function in pediatric patients with short bowel syndrome (SBS).
Takato Sasaki, Kouji Masumoto.
Citrulline, a non-proteinogenic amino acid, is essential in the synthesis cycle of nitric oxide and the urea cycle. It also acts as a precursor to arginine, a key player in amino acid metabolism and protein synthesis. citrulline is primarily synthesized in the small intestinal epithelium, and its endogenous levels are significantly higher than those derived from dietary sources. Given the predominance of endogenous citrulline, its levels could be a valuable marker for assessing the quantity of intestinal mucosa, thus indirectly evaluating its function. Short bowel syndrome, which results from massive resection of the intestine—either congenital or acquired—severely affects patient prognosis and quality of life, varying with the length and location of the remaining intestine. At our institution, we have analyzed plasma citrulline levels in several pediatric patients with short bowel syndrome. And, we also observed a fascinating relationship
between plasma citrulline levels before and after the administration of a glucagon like peptide 2 (GLP-2) analogue, a promising therapy for this condition, particularly in terms of reducing the necessity for parenteral nutrition. In this lecture, I will present these findings and discuss the potential of plasma citrulline levels as a biomarker for residual intestinal function in patients with short bowel syndrome.
 Alternations in Amino Acids and Lipid
Alternations in Amino Acids and Lipid
Metabolic Pathways in Cancer Stem Cells
KUNG-KAI KUO1, KAZUNARI K. YOKOYAMA2
1 Division of General and Digestive Surgery, Department of Surgery
2 Center of Stem Cell Research, Kaohsiung Medical University
Cancer recurrence after chemotherapy or radiotherapy is initiated by a subpopulation of residual malignant cells expressing stem cell markers and are believed to be cancer stem cells (CSCs). The CSCs are capable of self-renewal and can undergo differentiation to generate the phenotypic diversity observed in tumors. Recent studies have identified CSCs in hematologic, breast, liver, pancreas, and colon cancers. CSCs are not only the source of the tumor, but also responsible for tumor progression, metastasis, therapy resistance, and recurrence.
Redox adaptation significantly influences drug resistance. It is plausible that the drug-resistant CSC population employs redox regulatory mechanisms to enhance cell survival and tolerance to anticancer agents. Studies have shown that normal hematopoietic stem cells and mammary epithelial stem cells maintain lower levels of reactive oxygen species (ROS) compared to their mature progeny [Nature 2004;431:997-1002 Nature Medicine 2006;12:446-451]. This ROS reduction is associated with elevated expression of ROS-scavenging molecules, such as glutathione—an essential antioxidant amino acid. Interestingly, cancer cells exhibit higher ROS levels, which appear crucial for malignant progression. However, CSCs in human breast tumors maintain lower ROS levels than non-stemming tumor cells, potentially contributing to their stemness and cancer-forming capabilities [Hepatology 2013;58:218-228]. In a study by Trachootham, rapid depletion of glutathione using the natural compound PEITC not only eradicated Ras-transformed ovarian cells but also eliminated drug-resistant CSCs [Blood 2008;112:1912-1922]. This underscores the importance of redox regulation in CSC survival and therapeutic response.
The metabolism in CSCs is distinct from non-stemming cancer cells, which is highly dependent on glycolytic process (Warburg effect). But CSCs predominantly utilize oxidative phosphorylation (OXPHOS) pathways for ATP production and survival [Cell Stem Cell 2013;12:329-341 Blood 2015;125:2120-2130]. JAK/STAT3 regulates lipid metabolism through fatty acid beta-oxidation (FAO), which promotes breast cancer stemness and chemoresistance. Blocking FAO selectively eliminates breast cancer stem cells [Cell Metabolism 2018;27:136-150].
Yamanaka factors (Oct4, Sox2, Klf4, and Myc) were initially used to reprogram somatic cells into induced pluripotent stem cell-like cells (iPSLCs). In our lab, HepG2-derived iPS-like cells exhibited cancer stemness features and increased tumorigenesis after transplantation. Reprogrammed HepG2-derived CSC-like cells (rG2-DC-1C) showed continuous expression of stemness genes, chemoresistance, and invasion abilities. The sphere-colony formation ability and the invasion activity of rG2-DC-1C were also higher than those of HepG2 cells [Stem Cells, 2016;34:2613–2624]. By another way, we also successfully cloned drug-resistant strain, which expressed many CSCs’ markers and characters, from HepG2 cancer cell lines [Int J Mol Sci. 2022 Jul 14;23(14):7782].
In summary, understanding the metabolic alterations in CSCs is crucial for overcoming drug resistance. Targeting CSC-specific pathways may lead to more effective cancer treatments and therapies [J Exp Clin Cancer Res 43, 33 (2024)].
 Effects of glutamine and leucine on muscle injury and atrophy in sepsis
Effects of glutamine and leucine on muscle injury and atrophy in sepsis
Hou Yu-Chen
Associate Professor and Chair
School of Food Safety, College of Nutrition, Taipei Medical University, Taipei, Taiwan
Master Program in Food Safety, College of Nutrition, Taipei Medical University, Taipei, Taiwan
Abstract
L-glutamine (Gln), a nonessential amino acid, is found abundantly in the body and skeletal muscles. During severe illness, muscular Gln levels drop drastically, which is an indicator of skeletal muscle wasting. L-leucine (Leu) is an essential amino acid that supports the glutamate-glutamine pool in skeletal muscles by transamination. Our lab investigated the effects of Gln and Leu administration on skeletal muscle injury and atrophy in a mouse model of cecal ligation and puncture-induced sepsis. We also assessed whether the combination of Gln and Leu had synergistic effects. The results indicate that both Gln and Leu, when treated alone, not only alleviated sepsis-induced skeletal muscle damage but also attenuated muscle atrophy by reducing inflammation and protein degradation. Akt/mTOR signaling and expression of myogenic genes in muscles were enhanced by Gln and Leu. However, only Leu treatment upregulated the expressions of mitochondrial function-related genes. Gln and Leu combination failed to show synergistic effects on alleviating sepsis-induced muscle atrophy. These findings suggest that both Gln and Leu effectively reduced inflammation and skeletal muscle degradation during sepsis, with Leu treatment alone showing more pronounced effects in maintaining muscle mass.
 Dualism versus Monism in Surgical Nutrition
Dualism versus Monism in Surgical Nutrition
Jin-Ming Wu, M.D. & PhD
Clinical Associate Professor, Department of Surgery, National Taiwan University Hospital
Geriatric patients are the most growing population in the developed countries. The indication for major digestive system surgery is cancer, and the risk increases with aging. Therefore, geriatric patients are considered as the most common subjects receiving major surgery. It is more common to have multiple comorbidities and decreased physical reserve among older patients in comparison with younger ones. Although surgery is the main cornerstone in treating cancer, it deteriorates the physical condition and impair the quality of life. Strategies for fastening the postoperative recovery and strengthening the physical condition are very imperative to improve the outcomes.
Concerning the malnourished status in most geriatric cancer patients, nutritional intervention plays an important role to improve surgical outcomes and to complete chemotherapy course. However, during past decades, several concepts of nutritional support or intervention were proposed, whereas positive and negative ideas contradict each other. There are some examples, such as parenteral versus enteral feeding, over-feeding versus under-feeding, hyper-inflammation versus immunosuppression, and guideline-oriented versus personalized cares.
In addition to aforementioned modalities, precision medicine advocates to deliver the “right” drug to the “right” patient, and this trend had turned nutritional cares/intervention into “precision nutrition”. This field is moving at a rapid pace by combining pharmacological therapies and diet intervention. Its aim is to develop interventions to prevent or treat chronic diseases based on a person’s unique characteristics like gene, race, gender, health history, and lifestyle habits.
In modern era, the surgeons are facing more and more challenges. In this presentation, I will try to propose some views of elucidating these problems in nutritional support among surgical cancer patients. Future direction still needs further studies.








